The Single-Blind research method is a specific research procedure in which the researchers and those involved in the study do not tell the participants if they are being given a test treatment or a control treatment. When both do not know the treatment the study is double-blind.

Pin On Figure Drawing Paintingtube
This is done in order to ensure that participants dont bias the results by acting in ways they think they should act.

Single blind procedure definition. Medical Definition of single-blind. Definition of single-blind. Of relating to or being an experimental procedure in which the experimenters but not the subjects know the makeup of the test and control groups during the actual course of the experiments compare double-blind open-label.
The control does not receive this treatment but something in its place. A blind study is a clinical trial in which the subject or the Investigator or both are unaware of which trial productdrug the subject is taking. Of or pertaining to an experiment or clinical trial in which the researchers but not the subjects know which subjects are receiving the active treatment medication etc so as to eliminate subject bias.
A single-blind design is when the participant doesnt know if they are in the treatment group or the control eg. In a single blind peer review reviewers identities are kept hidden from authors. This is done to reduce the risk of errors since some participants might produce spurious results if they know that they are taking the placebo or medication.
This is the traditional form of peer review and its still the type thats most common. Eine Einfachblindstudie ist eine klinische Studie bei der nur die Verblindung der Studienteilnehmer erfolgt. In this model the experimenter monitoring the participants knows which individuals received the placebo and which.
A study in which both the experimenters and participants are unaware of who is receiving the independent variable and who. Psychology Definition of SINGLE BLIND. Single Eu Definition Of Blindness Could Help Save Billions Of Clinical Trial Design The Digital Cell Eurekalert Science News What Is Peer Review Single Blind Solved Question 2 Using Your Own Words Define The Follo Psych M D Double Blind Procedure The Neuron Placebo Response Rates And Potential Modifiers In Double Blind Doc Heyland Mqs Methodological Quality Score Annotated.
When only one is blinded to the data this is a single blind study. An experiment procedure where the people involved dont know of the treatment manipulation or type drug administered. In many studies the control group will receive a.
The blind in single blind review refers to what information authors can see. The treatment group in an experiment is the group that experiences the factor that the researchers hypothesize will have an effect. Of relating to or being an experimental procedure in which the experimenters but not the subjects know the makeup of the test and control groups during the actual course of the experiments compare double-blind open-label.
A clinical trial where the participants are unaware of. A technique for eliminating subjective bias as the placebo effect from the test results. You can also read about blind.
You can also add a definition of SINGLE BLIND yourself. An experiment procedure where the people involved dont know of the treatment manipulation or type drug administered. In psychology a single-blind study is a type of experiment or clinical trial in which the experimenters are aware of which subjects are receiving the treatment or independent variable but the participants of the study are not.
Single-blind definition of or relating to an experiment or clinical trial in which the researchers but not the subjects know which subjects are receiving the active medication or treatment and which are not. Dem durchführenden Prüfarzt ist die Zuordnung hingegen bekannt. In a single blind study the participants in the clinical trial do not know if they are receiving the placebo or the real treatment.
Die Testpersonen oder Patienten wissen nicht ob sie das Verum- oder das Placebo-Präparat erhalten. A testing procedure in which the administrators do not tell the subjects if they are being given a test treatment or a control treatment in order to avoid bias in the results.

Single Blind Vs Double Blind Peer Review What S The Difference
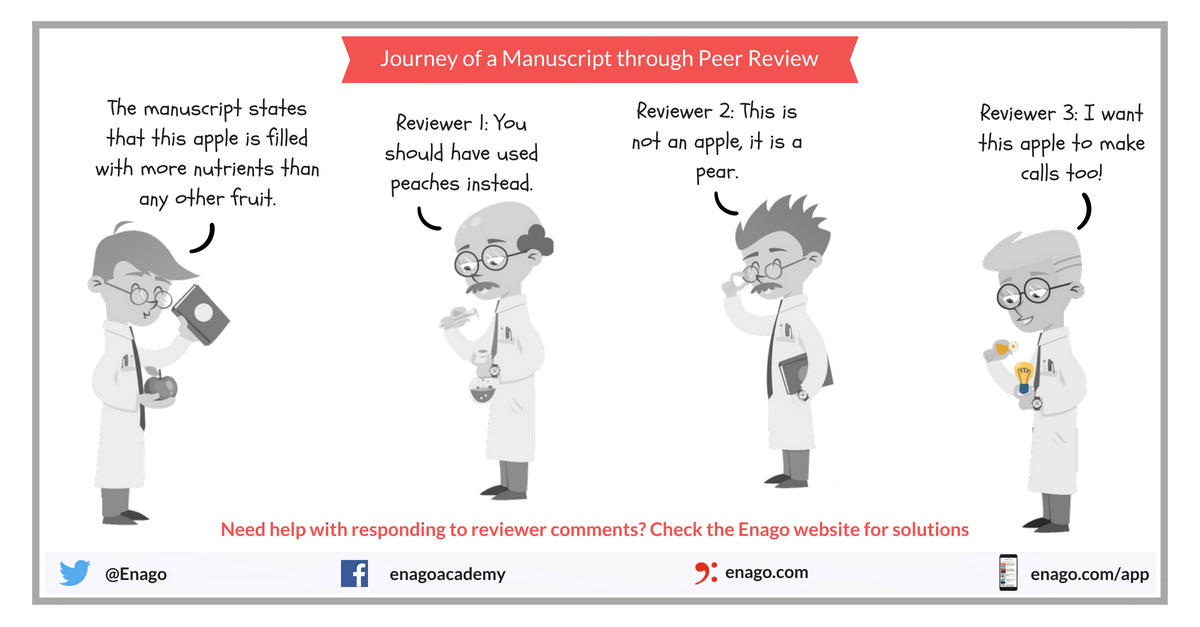
Single Blind Vs Double Blind Peer Review Enago Academy

What Is The Difference Between Single Blind And Double Blind Clinical Trials Biopharma Institute
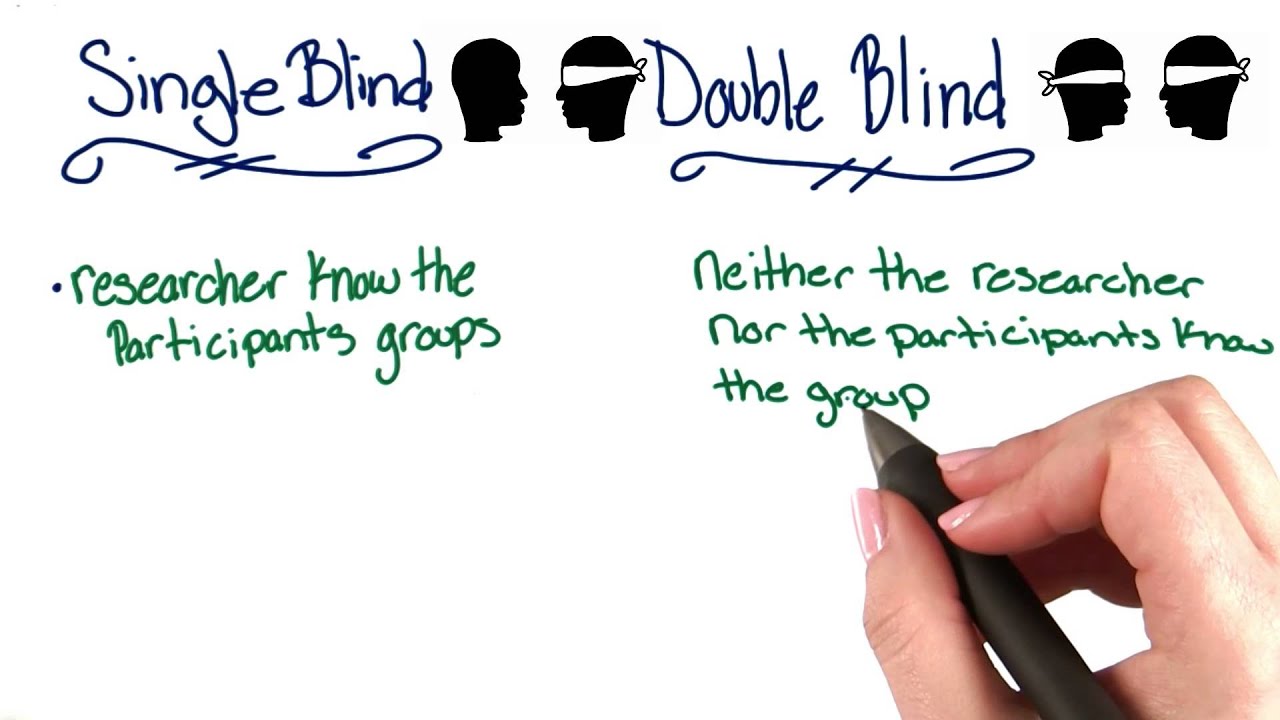
Double Blind Studies Intro To Psychology Youtube

Clinical And Biochemical Response To Single Infusion Of Clodronate In Active Rheumatoid Arthritis A Double Blind Placebo C Rheumatoid Arthritis Clinic Placebo

Single Blind Vs Double Blind Peer Review Enago Academy

What Is The Difference Between Single Blind And Double Blind Clinical Trials Biopharma Institute

Project Monthly Status Report Template 7 Templates Example Templates Example In 2021 Project Status Report Executive Summary Template Progress Report Template

The Full Form Of Bcc Stands For Blind Carbon Copy The Bcc Is Normally Used To Address The Electronic Conversation With The T In 2021 Form How To Apply Messages

Avid One Pager Old Yeller Middle School Reading School Reading Old Yeller

Rhetoric Cheat Sheet This Half Sheet Summarizes Rhetoric Specifically With A Definition Of A Rhetorical Situation The C Rhetoric Ethos Pathos Logos Cheating

Double Blind Studies Intro To Psychology Youtube

What Is The Difference Between Single Blind And Double Blind Clinical Trials Biopharma Institute










0 komentar:
Posting Komentar